Diabetes Ireland has launched its 2022 Pre-budget submission and is proposing 8 immediate actions for implementation which will improve the quality of life for over 225,000 people living with diabetes and reduce the long term costs of preventable diabetes complications. The submission is focused on a range of deliverable actions that are person-centred, cost effective and builds on existing HSE commitments to tackle chronic conditions including diabetes.
One of these Actions is access to a Flash Glucose Monitoring System
Flash Glucose Sensors (FreeStyle Libre®)
Flash Glucose Sensors (FreeStyle Libre®)
Adults over age 21 years, who manage their diabetes using insulin, either by multiple daily injections MDI or an insulin pump, cannot access the only Flash Glucose Monitor available in Ireland, the FreeStyle Libre®. This flash monitor offers a more comprehensive profile of glucose levels to help people with diabetes and clinicians to make more informed diabetes management decisions. We are asking that the government extend access to the Flash Glucose Monitoring (FreeStyle Libre®) to all people with diabetes on MDI or an insulin pump (Type1, Type 2, etc.) based on clinical need.
People with diabetes have been calling for the inclusion of Flash technology (FreeStyle Libre®) on the long-term illness scheme since 2016 when the device first became available in Ireland, the evidence of this can be seen in the hundreds of parliamentary questions submitted by TDs from then up to the present day. This did happen in 2018, but only for a very small cohort of the type 1 diabetes population and the community responded by organising a community led petition signed by 19,000 people, almost one signature for every person with type 1 diabetes in Ireland. Diabetes Ireland has supported the HSE in their review of the reimbursement scheme by submitting Irish clinical research and a user experience survey completed by 300 people self-funding their flash glucose monitors and yet we are still awaiting a decision on expanding the scheme two years later.
Flash (FreeStyle Libre®) Glucose Monitoring is one of the technologies designed to replace routine ‘finger-stick’ self-monitoring blood glucose (SMBG) for people with diabetes aged 4 or over, including pregnant women, who use multiple daily injections (MDI) or an insulin pump to deliver their insulin. These technologies also address many barriers with ‘finger-stick’ monitoring and their data sharing Apps allows clinicians, as part of virtual consultations, to effectively support people with diabetes under their care. This expanded use would provide a foundation to deliver efficient diabetes care post COVID-19 which can potentially reduce the need for face-to-face appointments. A recent Irish survey by Diabetes Ireland on the acceptability of virtual consultations with patients who took part in one during COVID-19 lockdown indicated that having this data saves time and leads to a deeper more productive consultation between the clinician and patient as there is greater understanding of glucose variability on which to base clinical decision making.
Furthermore, there is now a substantial body of clinical evidence including Randomised Controlled Trials (RCT), real world and observational studies demonstrating that the FreeStyle Libre® system safely improves clinical outcomes for people with diabetes. The Association of British Clinical Diabetologists (ABCD) FreeStyle Libre® UK Nationwide Audit involving 102 UK diabetes centres contributed, with over 10,000 users’ data collected, reported that FreeStyle Libre® system users had significantly less paramedic call outs, hospital admissions and episodes of severe hypoglycaemia in the 7.5 month follow up period – admissions for hypoglycaemia reduced from 120 to 45 and admissions for hyperglycaemia/ DKA reduced from 269 to 86, comparing 12 months pre–FreeStyle Libre® initiation to 7.5 months post. The cost of a hypoglycaemia admission in Ireland is in the region of €1000.
Irish based clinical evidence has been provided by a number of Irish diabetes centres plus a submission to the HSE (PCRS) by Diabetes Ireland which highlighted that adult with diabetes using the technology privately showed a 66% reduction in their blood glucose strip usage. This clinical evidence along with other analytical data provided by the PCRS was to be considered by the HTAG Review Team with a decision to be made by March 2020. Covid 19 has delayed this decision.
This mounting clinical evidence on the reduced hospitalisation costs and the massive improvements in the quality of life for people with diabetes should be a considerable factor in the decision to expand access to Flash Glucose Monitors.
Diabetes Ireland propose that funding for expanding access to people with diabetes be based on clinical need and be allocated from the estimated savings generated from the HSE Medicines Management Programme (MMP) evaluation to identify preferred blood glucose test strips which are estimated to be approximately €8-10m this year alone, and from offering choice to adults qualifying for Continuous Glucose Monitoring systems CGMs which share many of the features of the Flash (FreeStyle Libre®) but cost almost twice as much.
We estimate that the updated additional cost of Flash (FreeStyle Libre®) to be approximately €622 per person per year based on the 2017 HTAG and adjusted to reflect the February 2021 reduction in blood glucose monitoring strip price. If 4,000 adults were funded for Flash glucose monitoring in 2022 this would cost the HSE approximately €2.5 million. This does not consider any savings that could be made from potential reductions in hospitalisation for Flash (FreeStyle Libre®) users that have been seen in published clinical data.
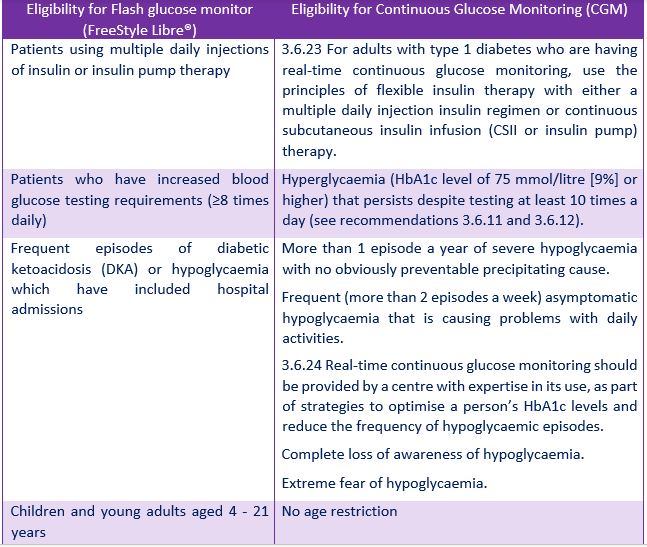
Additionally, we also have been made aware of the fact that the age restriction of 21 years applied to the Flash (FreeStyle Libre®) eligibility criteria has led to a 200% annual increase in uptake of continuous glucose monitoring CGM in this age group since 2018. Both devices share many of the clinical need criteria but a CGM costs €1,000 more per person per year. This trend appears to be continuing in 2021. If Flash (FreeStyle Libre®) was an option for 1,000 people with diabetes in this age group, we estimate that the HSE would save €1.18 million annually on its current expenditure by removing the age restriction. This brings the total estimated cost of expanding Flash (FreeStyle Libre®) to 4000 people to €1.32 million in 2022. See Table 2 for a comparison of eligibility criteria between the two technologies:
Diabetes Ireland is calling for Flash (FreeStyle Libre®) to be made available to all people with diabetes, who use MDI or an insulin pump (Type1, Type 2, etc.) to manage their diabetes based on clinical need and is asking that savings from the blood glucose strips be earmarked for adults who are deemed to clinically require the Flash (FreeStyle Libre®) by their diabetes teams and offset by the savings from offering choice. This will improve quality of life for the individual and further aid prevention of costly diabetes complications and make short and long-term savings for the health service.
Diabetes Ireland contacted all TDs and Senators on Wednesday 14 July on behalf of our community asking them to make representations on this and the other issues contained in our Pre-budget submission. Some of our active volunteers’ groups also followed up locally with their own local representatives. We are now asking you to Act. We need your help to highlight this issue once again in advance of the upcoming budget.
Call for Action: What Can I do?
- Copy the template below into an email and if you wish add your personal story and reasons why this problem matters to you.
- Find your local TD’s (constituency) https://www.oireachtas.ie/
- Send your email to your local politicians.
Email/Letter Template
Dear (INSERT NAME OF TD)
I am a constituent and regular voter in your area.
I/my family member have/has lived with [INSERT THE TYPE OF DIABETES] diabetes for [INSERT YOUR DIABETES DURATION] years and for years I/my family member have/has been checking my/his/her blood sugar levels by finger-pricking. [INSERT WHY IT IS A STRUGGLE FOR YOU, WHY WOULD YOU LIKE TO USE TECHNOLOGY, AND WHY YOU ARE NOT USING IT YET]. (You can write here your personal story and the reason why you would like to highlight this particular issue to politicians).
Flash Glucose Monitoring (FGM, Libre) is currently only reimbursed for children and young adults under 21 years of age in Ireland. Although health-care professionals prescribe Libre based on the clinical need for many people with diabetes over the age of 21 years, [you can add if it was your case], patients are frequently denied access under the LTI reimbursement scheme.
Diabetes Ireland is asking that the government extend access to the Flash Glucose Monitoring (FreeStyle Libre®) to all people with diabetes on MDI or an insulin pump (Type1, Type 2, etc.) based on clinical need. I support this as I agree that age should not be a barrier.
As this topic is very close to my heart, I am writing to you to ask for your support by asking the Minister for Health to remove the current age restrictions for the Freestyle Libre.
Parliamentary Questions to ask the Minister for Health:
- To ask the Minister for Health if he has plans to provide funding in the upcoming budget to fund the provision of the Freestyle Libre Flash Glucose Monitoring System to people with diabetes over 21 years of age based on clinical need and if he will make a statement on the matter.
- To ask the Minister for Health if he will ringfence some of the anticipated 2021 cost savings from the recent HSE Medicines Management Programme (MMP) Preferred Blood Glucose Strips for adults with Type 1 and Type 2 diabetes evaluation to fund the provision of the Freestyle Libre Flash Glucose Monitoring System to people with diabetes over 21 years of age based on clinical need and if he will make a statement on the matter.
- To ask the Minister for Health how many applications for Freestyle Libre were submitted by individual hospitals for people with diabetes over 21 years of age; how many were approved; how many were refused in each of the years 2017-2021 in tabular form; are there plans to remove the age restriction on Freestyle Libre and if he will make a statement.
For more detailed information about this issue, please see here: https://www.diabetes.ie/advocacy-call-to-action/access-to-a-flash-glucose-monitoring-system/
Thank you for your time in considering my request.
I hope to hear from you in due course.
Yours sincerely,
To read the full Pre Budget Submission, click here.
Please note there are many other important issues we plan to highlight and advocate for going forward. implementation and we will be working with all stakeholders to do this in a strategic way.
Our advocacy work is important in giving our diabetes community a voice to be heard, become a member today, click here.
To read the next call for action to deliver specialist community diabetes teams , click here.
Call to action July 2021